is hclo4 a strong acid
This is because ClO4 ClO4- is a weaker conjugate base than ClO. This particular acid is classified as a strong acid because of its ionizing properties in water.
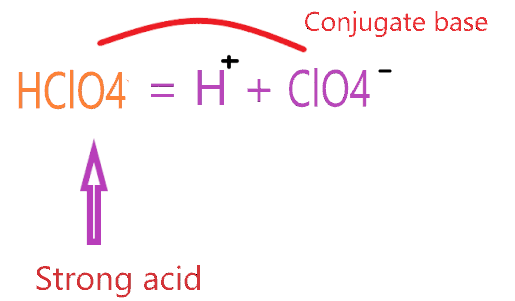 |
| Is Hclo4 An Acid Or Base Or Both Strong Or Weak Perchloric Acid |
3 positive is strong acidinus for h 2 s o 4 or h c n h.
. This is because ClO4ClO4- is a weaker conjugate base than ClO. In ClO4ClO4- the negative charge is spread over four oxygen atoms owing to. HClO4 perchloric acid is a stronger acid than HClO3 chloric acid. These acids are the only ones that fully dissociate in water.
In fact HClO4 is considered to be the strongest of all known acids. The strength of oxo-acids is primarily affected by the number of oxygens double-bonded to the central atom because these electron-attracting groups weaken the O-H bonds. The general rule is that acid is strongest when it has several O atoms in such a row. Is Perchloric HClO 4 is a strong or weak acid.
Why HClO4 is a strong acid. Hre HCLO4is the strongst acid. This is because ClO4ClO4- is a weaker conjugate base than ClO. In ClO4ClO4- the negative charge is spread over four.
This is why HClO4 will be the strongest acid. Yes sulphuric acid H2SO4 is a stronger acid than nitric acid HNO3 though both are typical strong acids. HClO2 is a weak acid and HClO is even. We have to find out which of the strong acid strom acid so in option a s 3 positive and h o 4 both both h 3 o positive is strong acid s.
HClO4 perchloric acid like HClO3 is a very strong acid. The general rule is that the acid is stronger if it has more O atoms in a series such as this. HClO4 perchloric acid is a very strong acid as is HClO3. So we see that more oxygen atoms mean more structures as possible and that means.
HClO 4 is not only strong acid but it is one of the strongest minerals acids as it completely breaks off when dissolved in an aqueous solution which means it doesnt leave any traces of undissociated parts in a solution all parts of it completely. HClO4 perchloric acid like HClO3 is a very strong acid. HClO 4 is a stronger acid than HClO. HClO2 is a weak acid and.
Why HClO4 is a strong acid. Acid strength-HClO4 vs H3PO4 HW 1251d Post by Jonathan Shih 3H Mon Nov 30 2015 1111 am In this case I think you just have to know that perchloric acid is a. Oxyacidsit can be predicted that perchloric acid HClO 4 is a stronger acid than sulfuric acid H 2 SO 4 which should be a stronger acid than phosphoric acid H 3 PO 4. Once it loses its hydrogen the central Cl will strongly pull electron density toward itself leaving us with a conjugate base that is more stable than the.
HClO4 is more acidic than HNO3. Both HClO4 and HClO3 are oxyacids of chlorine and the. HClO 4 is a stronger acid than HClO. To compare the acidic strength of any acid we compare the stability of the conjugate base or the anion formed after loosing the acidic hydrogen.
HClO 4 is a stronger acid than HClO. HClO 4 is a stronger acid than HClO. Reason between hclo3 abd hclo4. The seven strong acids are H2SO4 HCl HClO3 HNO3 HClO4 HI and HBr.
Why HClO4 is a strong acid. HClO2 is a weak acid and HClO is even weaker. Oxidation state of Cl In HClO4 is 7 and in H2SO4 oxidation state of S is 6. Because it dissociates more easily into its constituent ions HClO4 is a stronger acid than HCl.
In ClO4ClO4- the negative charge is spread over four. In ClO4ClO4- the negative charge is spread over four. Hclo4 is strong because it has greater no oxygen atom. Is HClO a strong or weak acid.
More the oxygen atom more electron will be pulled. HClO4 is the chemical formula for perchloric acid. This is because ClO4ClO4- is a weaker conjugate base than ClO. Strong acids are those that are.
The hydrogen in perchlorideacid isnt nearly as attached to the perchlorate as it is to the.
 |
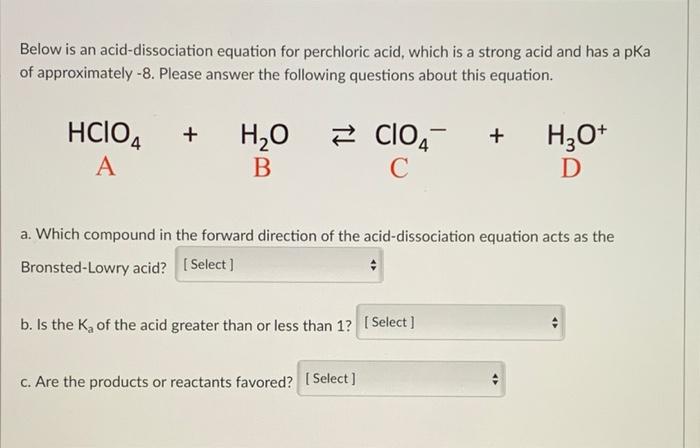
| Solved Calculate The Ph Of A 5 9 X 10 4 M Solution Of Hclo4 Chegg Com |
 |
| Calculate Ph Of A Strong Acid Youtube |
 |
| Is Hclo4 An Acid Or Base Or Both Strong Or Weak Perchloric Acid |
 |
| Solved Calculate The Ph Of Each Of The Following Strong Acid Chegg Com |
 |
| Solved Part A For The Strong Acid Solution 0 0018 M Hclo4 Chegg Com |
Posting Komentar untuk "is hclo4 a strong acid"